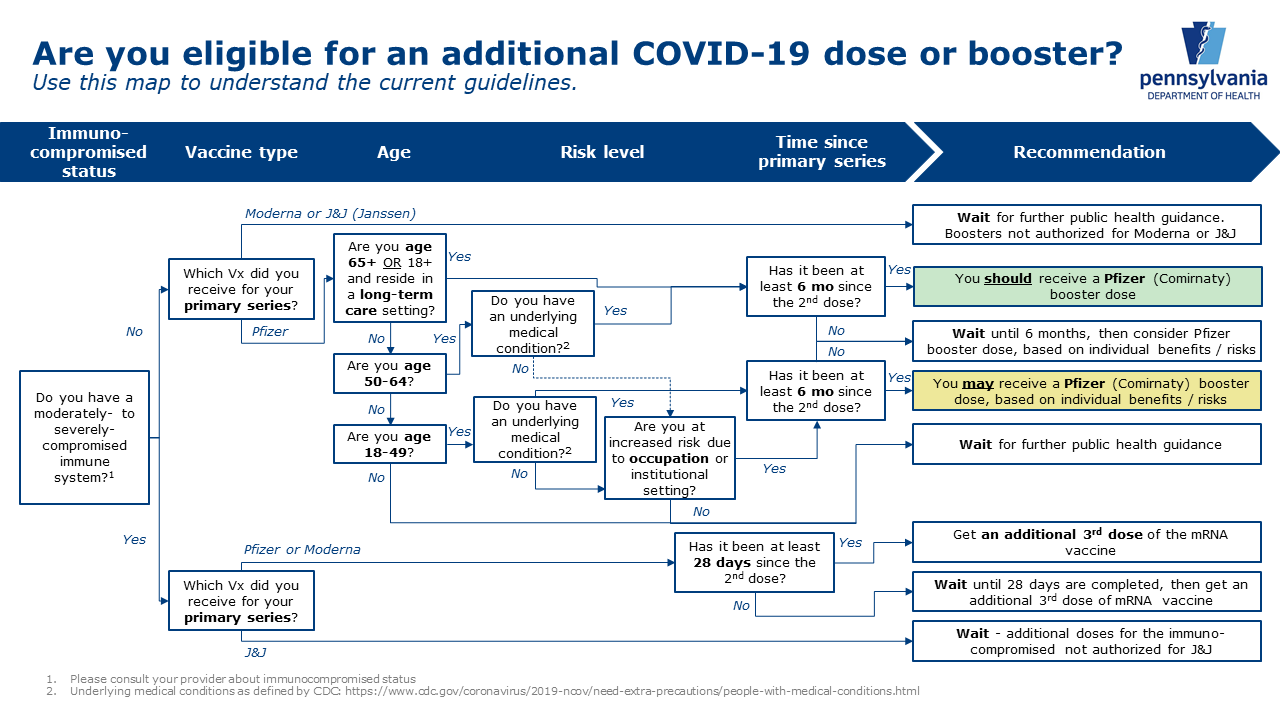
U.S. FDA on X: "Today, we amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 Vaccine to allow for use of a single booster dose, to be administered at least 6

FDA authorizes another booster dose of the Pfizer or Moderna COVID-19 vaccine for people age 50 and up - The Boston Globe
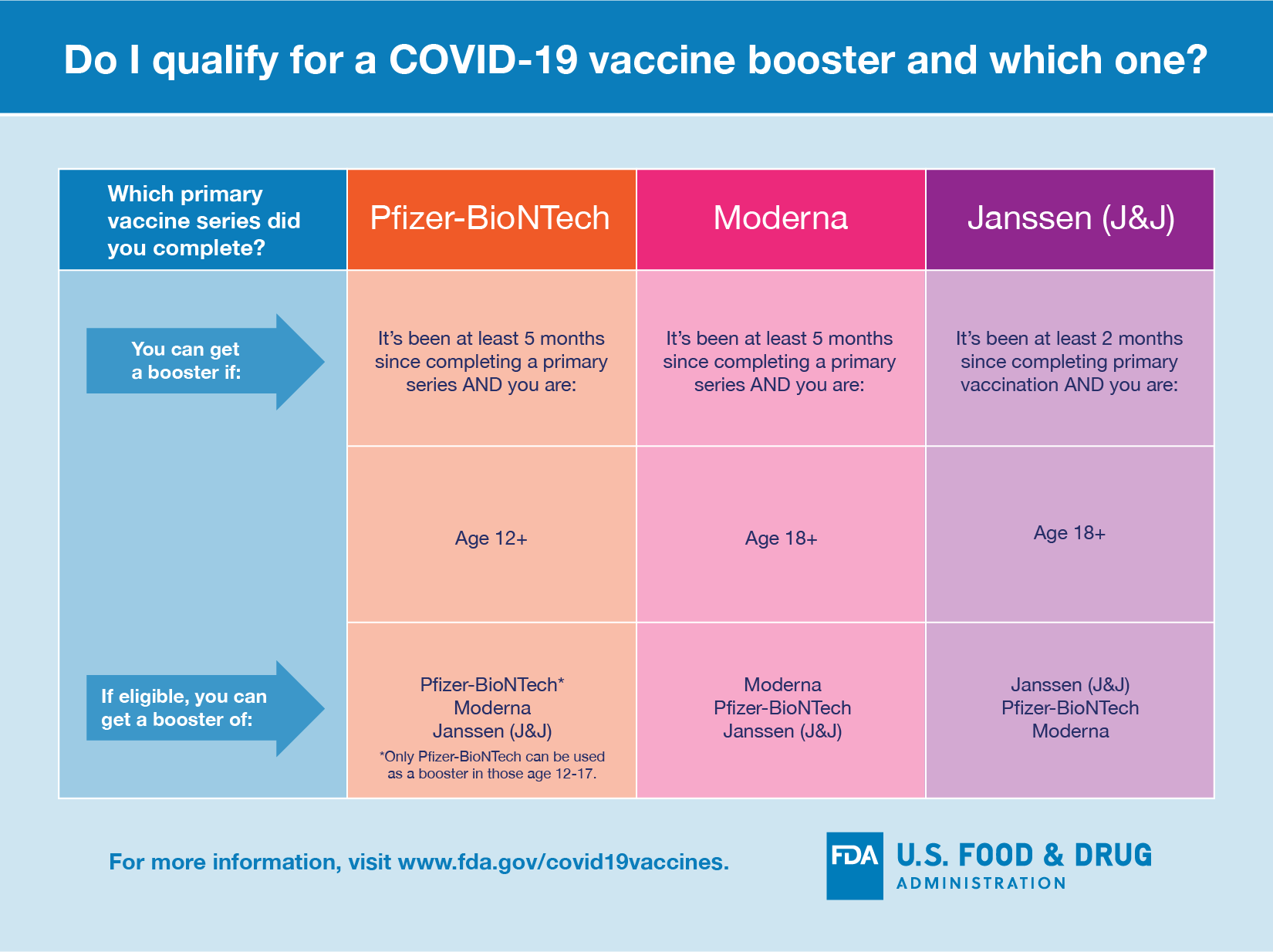
Coronavirus (COVID-19) Update: FDA Shortens Interval for Booster Dose of Moderna COVID-19 Vaccine to Five Months | FDA

FDA authorizes new guidance on Pfizer vaccines and boosters for children and adults : Oregon Health News Blog

Plans for Vaccine Booster Shots from Pfizer and Moderna Being Finalized Now by FDA, CDC – AZ Dept. of Health Services Director's Blog

FDA Panel Says Pfizer COVID Booster OK For Older People And Those At High Risk : Coronavirus Updates : NPR