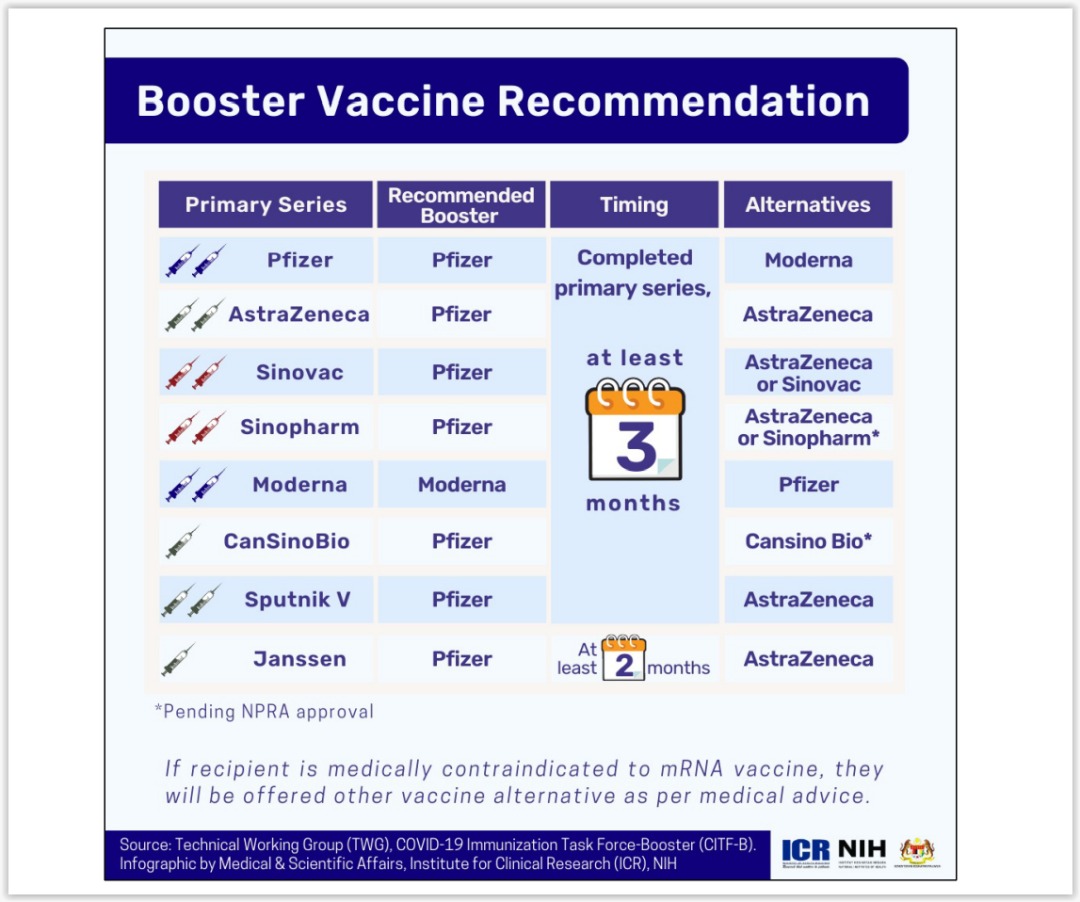
PR Thai Government - The Center for COVID-19 Situation Administration (CCSA) has approved shortening the period of the third dose, or booster dose, to tackle the COVID-19 Omicron variant, which is spreading

Department of Health (Philippines) - All fully vaccinated adults (18 years old and above) are now eligible to receive single-dose booster shots at least three months after the second dose of the

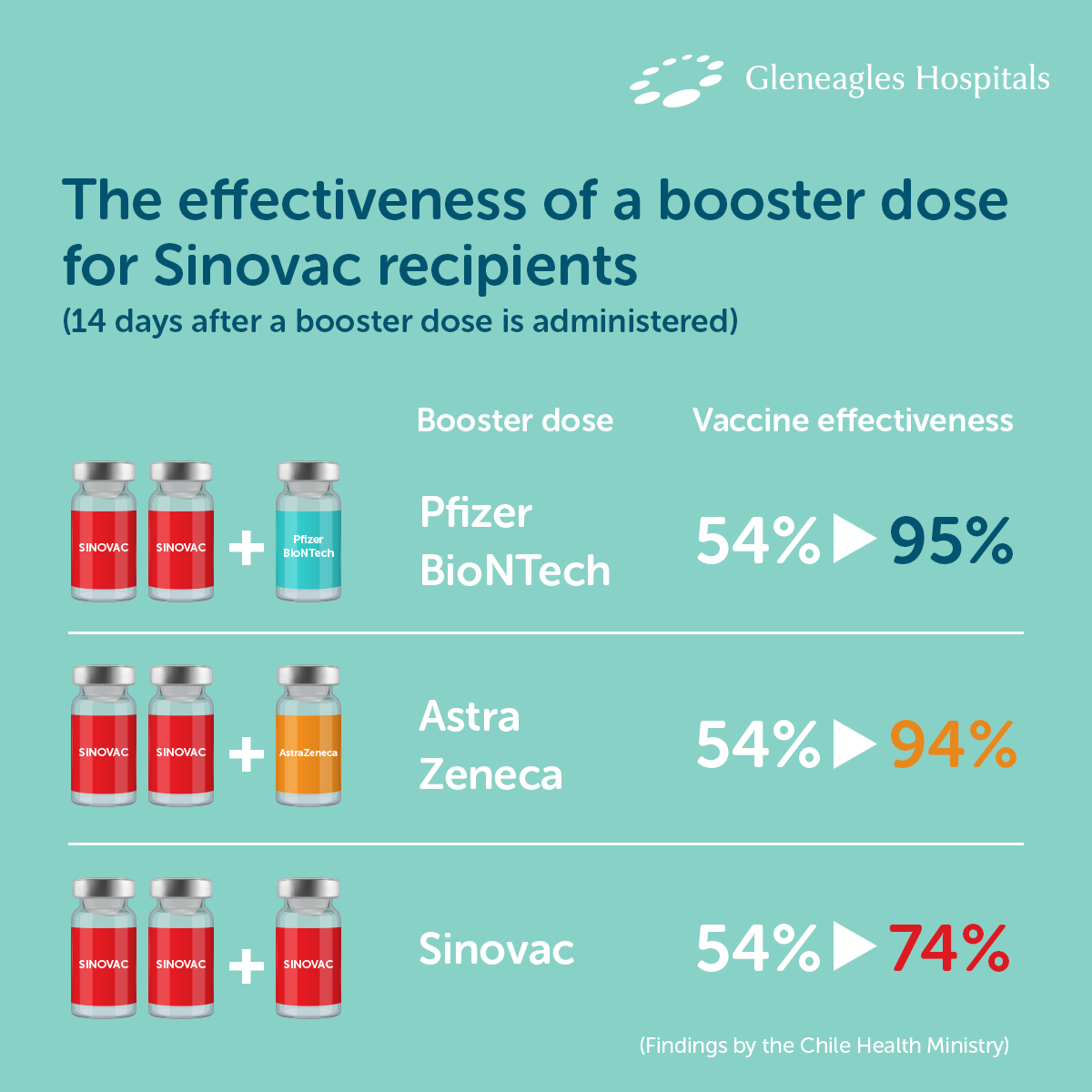
Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK - The Lancet

COVID-19 Vaccine Booster Shots (3rd Dose)|Ministry of Health, Labour and Welfare, government of Japan|厚生労働省

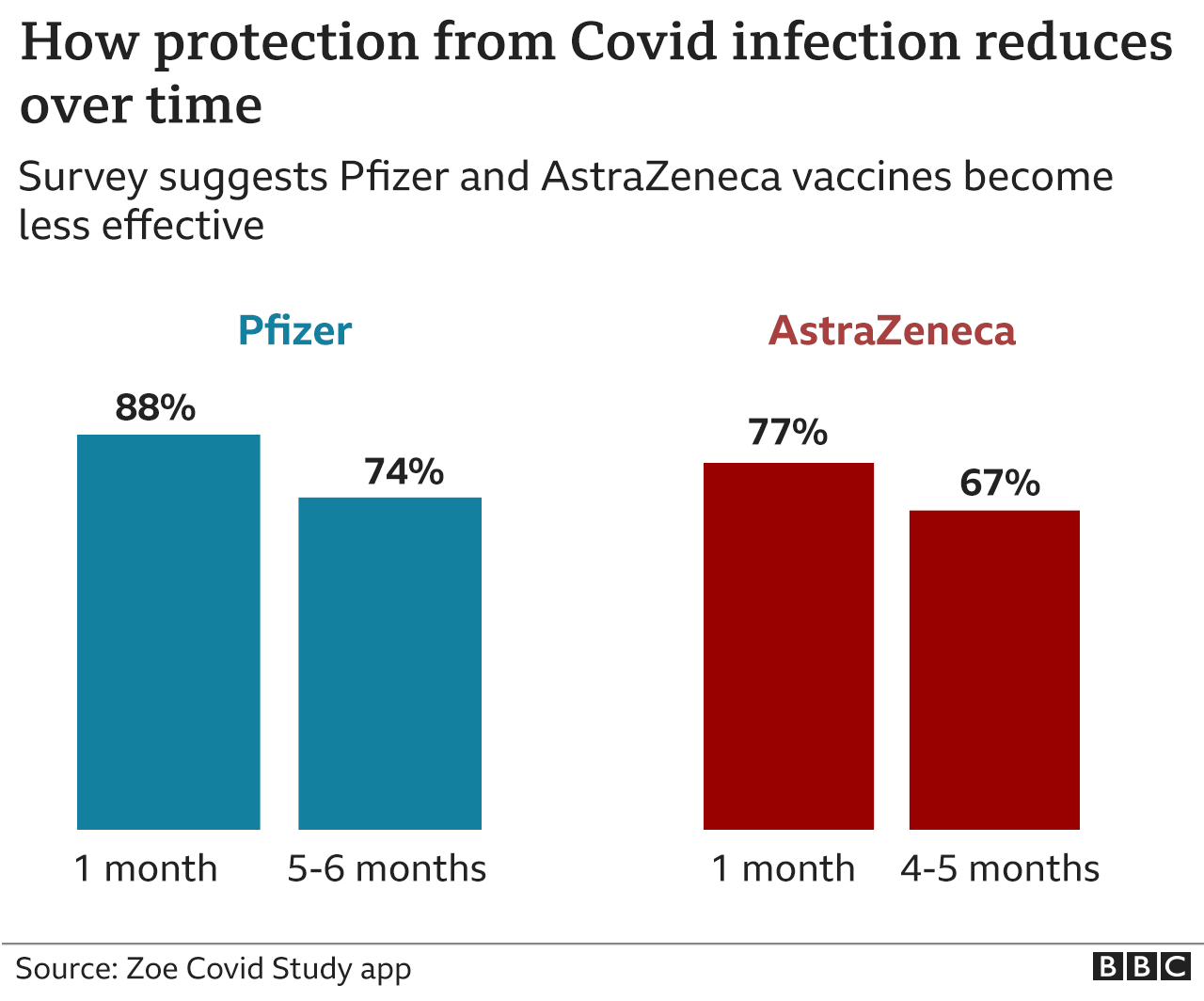
COVID-19 vaccine waning and effectiveness and side-effects of boosters: a prospective community study from the ZOE COVID Study - The Lancet Infectious Diseases

Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials - The Lancet
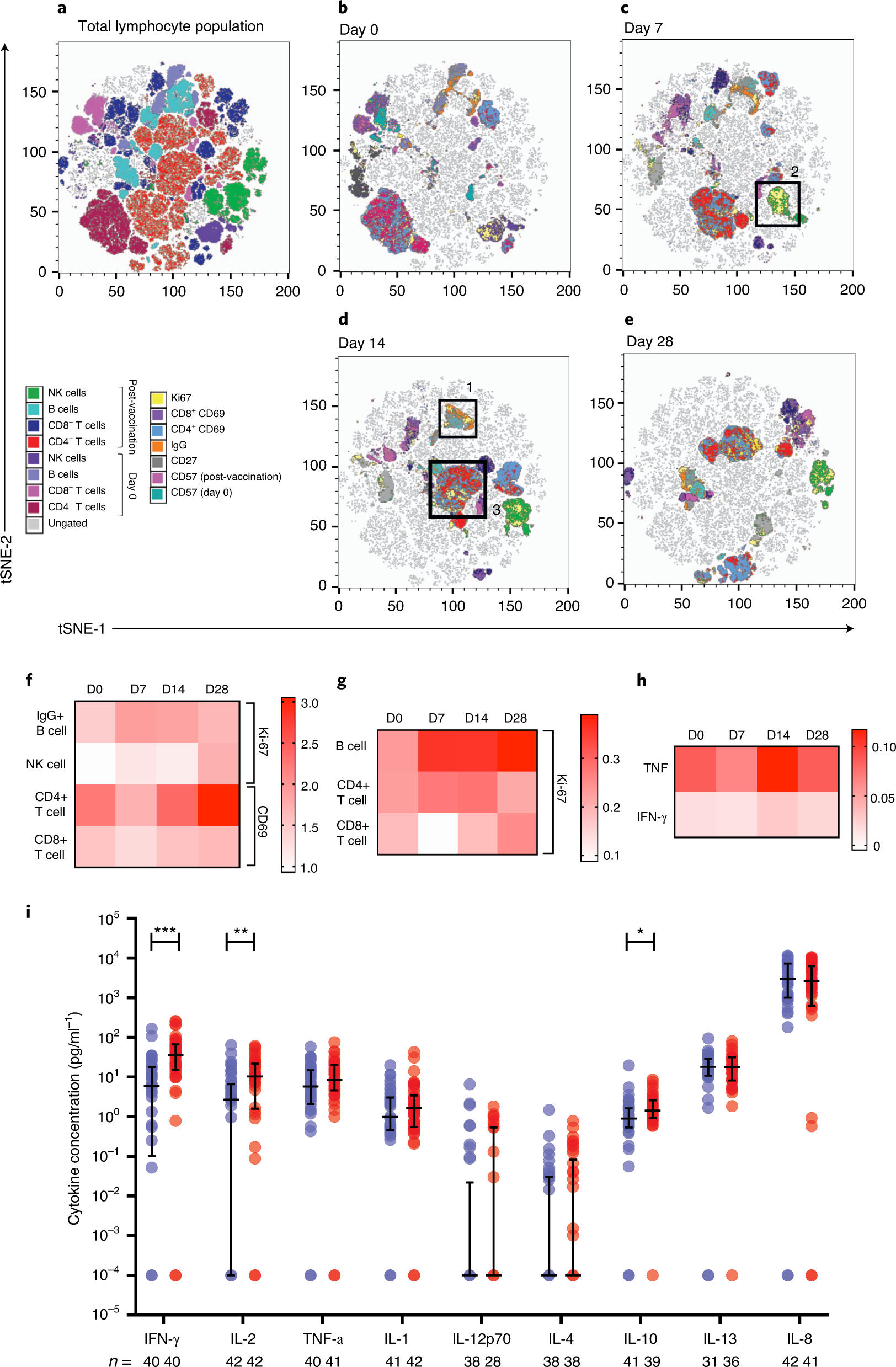
T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial | Nature Medicine